Buy Photo Prints from the Best Image Archives
Welcome to your one-stop destination for high-quality and affordable photo prints and wall art. We offer you a selection of high-quality photo prints that capture the beauty and diversity of the world around us, with state-of-the-art printing that ensures every photo print is of exceptional quality and detail.
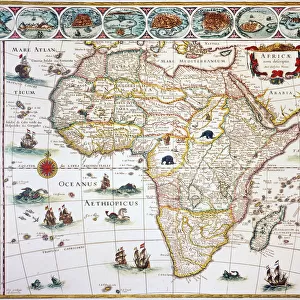
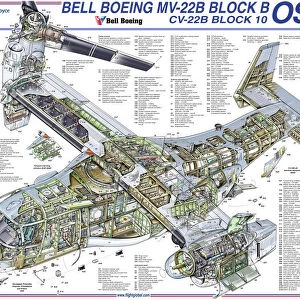
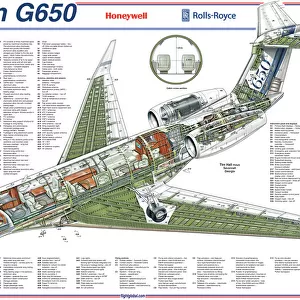
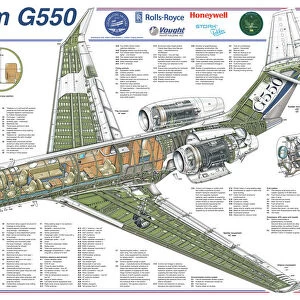
Our archive of images is carefully curated to bring you a wide range of subjects, including Landscapes, Animals, Architecture, and much more. We pride ourselves on offering photo prints in a variety of sizes and finishes to suit your preferences and style, including glossy, matte, and canvas.
Plus many more...Our Products
Framed and Canvas Prints plus a lot more.
Turn your chosen image into high quality Wall Art, Homeware, Gifts and Greetings Cards
Discover Our Collections
Find your Perfect Print... it's simple!
select_all Choose your Image
Explore our library and find the print you've been looking for. With over 150 brands, you are sure to find a piece you'll love
auto_fix Choose Your Product
Our beautiful Wall Art and Photo Gifts include Framed Prints, Photo Prints, Poster Prints, Canvas Prints, Jigsaw Puzzles, Metal Prints, and so much more
departure_board Printed and Shipped
Professionally printed for superior quality or your money back. Carefully packaged to arrive quickly and safely

Latest News
How do I buy Photo Prints?
Buying Photo Prints is a simple process. You can search our vast selection of photos by keyword or browse through categories such as Landscapes, Animals, and Sports.
Once you have found a photo that interests you, click on it to view more details about it including its size and price. You can also choose whether or not to add any additional features such as framing or mounting options before adding it to your shopping cart.
After selecting all of the photos that you would like to purchase, proceed to checkout where you will be asked for shipping details and secure payment information.
Once your order has been processed successfully, we will send out your prints within 1-2 business days via standard shipping services depending on what is available in your area (please allow extra time for Mounted and Framed Prints). Your prints should arrive a few business days after they have been shipped out depending on where they are being sent from and how far away they are going.
Buying photo prints is a straightforward process that anyone can do in just a few minutes!
How much do Photo Prints cost?
Photo Prints vary in cost depending on the size and type of print you are looking for. For larger print sizes our Poster Prints may offer a cost effective alternative. If you are looking for a canvas wrap or metal print, these will be more expensive depending on the size you choose.
We quote shipping rates at the checkout so you can easily group multiple prints into one order to help optimise your shipping costs.
Overall, archive quality Photo Prints are very reasonably priced and there is something available for everyone's budget!
How will the Photo Prints be shipped to me?
Depending on your location, you can choose from a variety of shipping options.
We try to automatically default to the correct store for your location, you can also manually choose from our USA, UK, Europe and Australia stores at the base of the page - useful if purchasing for friends and family overseas.
If you are located in the United Kingdom, you can select Royal Mail or Courier Delivery. Royal Mail is an affordable option that typically takes 2-3 working days for Photo Prints delivery. Courier Delivery is a more expensive option but it can allow next working day delivery if ordered before midday.
If you order from our USA, European or Australia stores we will aim to ship directly from one of our partner print labs located in the USA, EU (Netherlands) and Australia for quicker and easier shipping. Most of our shipping options are tracked so you can check your orders progress. We also ship globally and we would recommend visiting whichever store is located closest to you.
No matter which method of shipping you choose, we ensure that all orders are securely packaged to ensure safe arrival at your location.
What can I use Photo Prints for?
Photo prints are a great way to capture and preserve special moments in life. They can be used for a variety of purposes, from decorating your home to creating keepsakes for loved ones.
One of the most popular uses for photo prints is to decorate your home or office space. You can choose from a variety of sizes, styles, and finishes to create the perfect look for any room. Whether you're looking for something modern or classic, you'll be able to find something that fits your style perfectly. And with so many options available, you'll be sure to find something that suits your budget too!
Another great use for photo prints is as gifts or keepsakes. Whether it's a special occasion like an anniversary, retirement or birthday, or just because you want to show someone how much they mean to you - photo prints make wonderful presents that will last forever! You could even have them framed and hung up on the wall as an extra special touch.
What is the cheapest way to buy Photo Prints?
Our Archive quality Photo Prints are very reasonably priced and there is something available for everyone's budget. We have kept our prices permanently low to bring you a unique collection of pictures at reasonable cost. If you are in a hurry, or simply wish to keep costs down, you can order a Photo Print and frame it at home. 10x8 and 20x16 Photo Prints are popular sizes, as are A2 Posters for larger prints. It should be reasonably easy to find frames in these sizes.
If you are placing the print into an off-the-shelf frame bought elsewhere please take into account that the original artwork may not be the same shape as your frame and when ordering you have the option to Zoom In and crop the picture so that it fills the paper. You can adjust the cropping in the Shopping Cart as it may not be suitable for all images and varies with personal preference. As part of our service, our own Framed and Mounted Prints are individually custom cut for each print to make sure that no unsightly margins are visible.
When ordering multiple prints we recommend placing them all in one order to optimize your shipping costs. To get your order to you as quickly as possible our automated systems mean that multiple orders cannot be combined into a single delivery at a later stage.